

Ultrasonic Tissue Ablation
For Integra Lifesciences
scroll to learn more
Services
Electrical Engineering
Embedded Software Development
Awards
CE and CSA Mark approved
FDA and CE Mark cleared
CUSA® Clarity on market
Integra LifeSciences Receives FDA (510K) Clearance for Specific Neurosurgery Indication for CUSA® Clarity
Delivering on a tight timeline
Integra LifeSciences Corporation engaged with Sunrise to support the development of the next generation Ultrasonic Tissue Ablation system. Sunrise Labs designed and developed electronics and firmware for real-time control of an aspiration and irrigation subsystem on a fast-paced timeline.
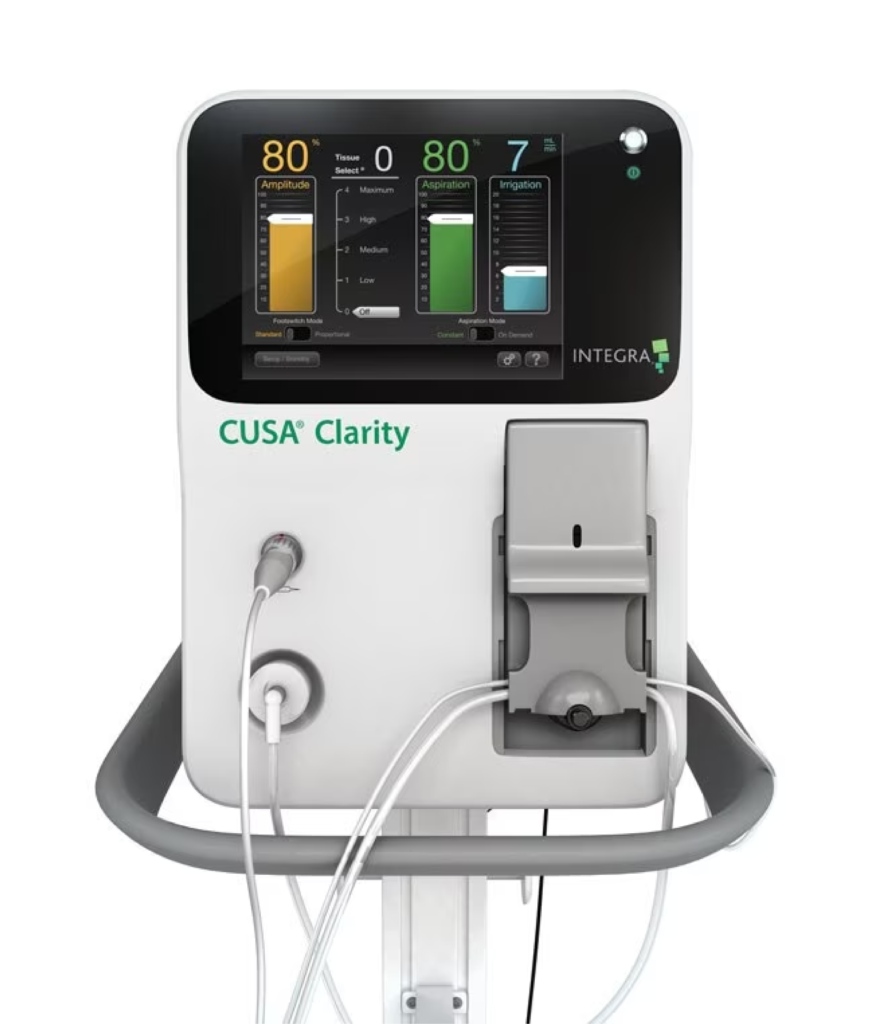
Reduced surgery times and improved patient outcomes
CUSA® Clarity provides surgeons with more control when performing ablation procedures. This allows surgeons to differentially ablate tissue, leaving vasculature in place while removing surrounding structures.
Challenging accuracy requirements were complicated by an aspiration pump which would slowly wear out over time. Sunrise’s control system design anticipates and accounts for this known failure mode. Surgeon expectations were met for this industry-leading high-frequency surgical device. The combination of power and precision can reduce surgery times and improve patient outcomes, helping the product exceed sales expectations. The electronics were also designed with multiple modular boards, including a Windows Qseven module, to support future feature enhancements and extend product life.
The resulting design meets IEC 60601-1 and IEC 60601-2-2 standards for the safety of high-frequency surgical equipment. CUSA® Clarity received CE mark and FDA 510(k) approval.
Let’s Work Together
Let us help you solve critical challenges to change people’s lives. Partner with our team of experts passionate about applying technology to improve lives.




