Work for us
Sunrise Labs develops medical devices that save and improve lives.
Revolutionary companies trust us to solve their toughest design challenges, and that’s what we do at Sunrise; solve design problems; for one product after another.
We seek and employ talented, enthusiastic engineers and product development managers. Our high integrity and supportive culture Sunrise invests in its people, offering generous benefits and competitive salaries.

Best Company to Work For
Sunrise Labs celebrates ‘Hall of Fame’ status from Business NH Magazine’s Best Company to Work For competition
By sticking to our purpose and mission to make lives better, and living our core values of respect for each other and integrity in all we do, Sunrise stands out as a wonderful place to learn and grow. “This honor is an acknowledgment that our employees are deeply engaged in the exciting and meaningful work we do for our clients,” says CEO, Eric Soederberg.







Sunrise Labs might be the place for you if you…
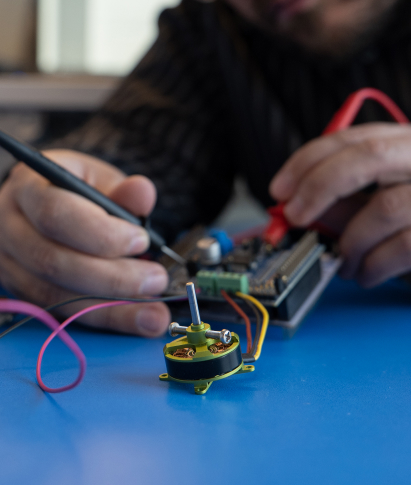
Want to work on important technologies that improve and save lives
Have a true passion for software development, electrical or mechanical engineering, medical systems engineering, or user experience design


Thrive on challenges in a fast-paced, high-integrity, and high-respect environment
At Sunrise we co-create value with and for all of our Stakeholders
Our People
We are recognized for our People’s Development in a Supportive High Respect Environment.
- Everyone is empowered and accountable (think agile)
- We provide Opportunities for Growth
Our Clients
We provide Agile Concierge Services to our Clients. Partnerships to make lives better. Focused on Best in Class:
- Engineering Excellence / Design
- Project Management
- Systems Integration
Our Communities
We lead socially responsible practices across Sunrise and in our communities.
- Social and Environmental respect; promotion of renewable energy use
- We support local schools, nonprofits, and charities through sponsorship of fundraising events, volunteer time, and mentorship
Our Location
We offer flexible and remote work options for most positions. The majority of our people choose a hybrid remote/in-office work experience. This provides for collaboration on project design while meeting each employee’s work environment needs.

We are headquartered in southern New Hampshire where the mountains, lakes, and ocean are our playground, and Boston is just down the road.
Our Openings
EEO Statement: At Sunrise Labs, we are committed to providing an environment of mutual respect where equal employment opportunities are available to all applicants and coworkers without regard to race, color, religion, sex, pregnancy, national origin, age, physical and mental disability, marital status, sexual orientation, gender identity, gender expression, military and veteran status, and any other characteristic protected by applicable law. Sunrise believes that diversity and inclusion among our coworkers are critical to our success as a design consulting company, and we seek to recruit, develop and retain the most talented people from a diverse candidate pool.
We do not accept resumes from staffing agencies or search firms unless a valid search agreement is in place with the agency for a specific position.
We have several levels of engineering opportunities and are always interested in starting a conversation with talented individuals like yourself. Send your resume to personnel@sunriselabs.com, so we can see if there is an opportunity for you here!
See what our team has to say
Drew Sunstein
Chairman
Dillan Murray
Designer/Technician
Eric Soederberg
President
Michael Peret
Technician
Nick Lesniewski-Laas
Director of Electrical Engineering



