
RFID Breast
Tumor Localizer
For Health Beacons
scroll to learn more
Services
Electrical Engineering
Embedded Software Development
Mechanical Engineering
UX/UI Design
Project Management
Awards
Achieved FDA 510(k) clearance
Health Beacons, Inc acquired by Hologic, Inc
Elevated to Success
Sunrise partnered with Health Beacons to transform their early prototypes into a full product using our 13485-certified process. Health Beacons was later acquired by a major strategic investor, Hologic, after a successful product launch.

Benefits for Clinicians and Patients
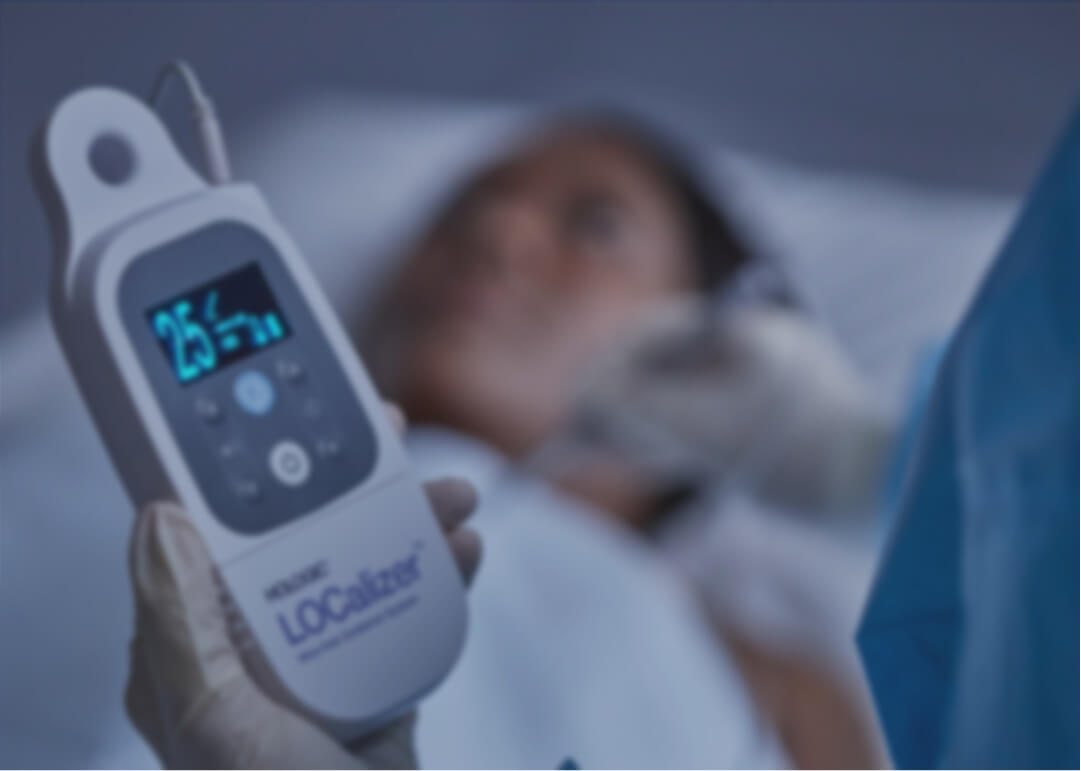
Health Beacons sought to commercialize their RFID Breast Tumor LOCalizer™ prototype. Sunrise transformed it into a user-centric, fully-tested and documented product for FDA market submission. With its unique, ergonomic, and portable design, the LOCalizer™ reader is suitable for use in both sterile and non-sterile environments.
LOCalizer™ is a wire-free guidance system that uses miniature radio frequency identification (RFID) tags to mark and guide physicians to non-palpable breast lesions. The tiny tag can be placed any time prior to or on the day of surgery, making scheduling convenient for both clinicians and patients.
Sunrise Support
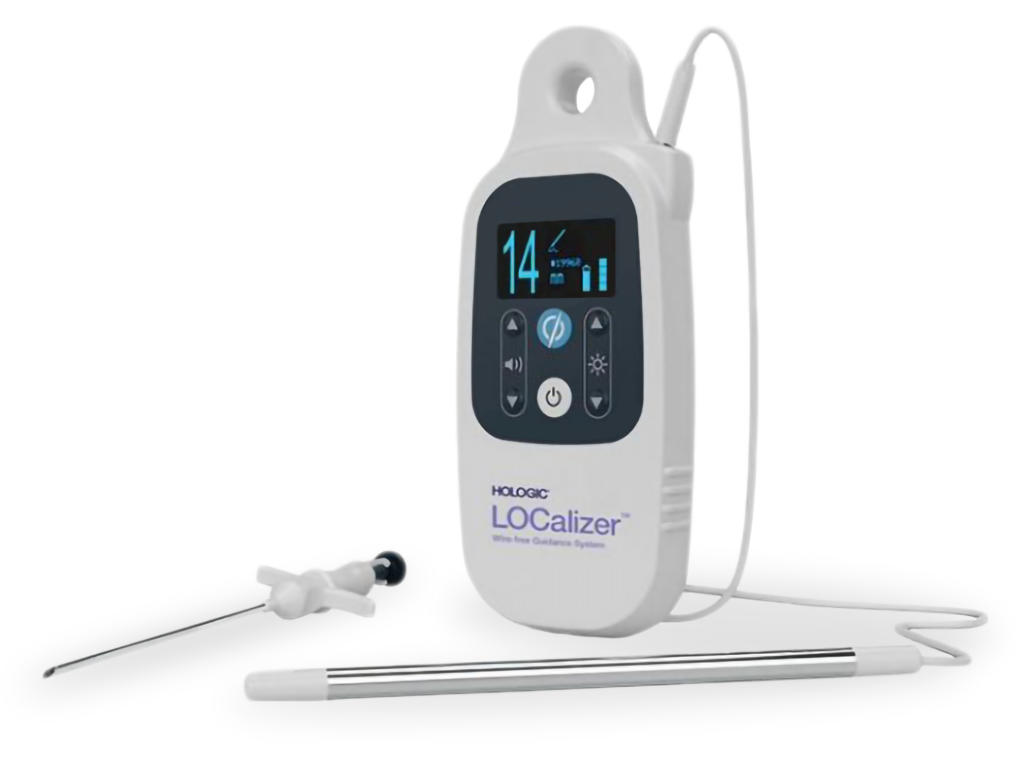
Sunrise provided support and overall management through the entire product development journey to validate and verify the system. Obsolete components were removed and new processors, sensing coiling, and display with customized iconography were added. Testing and verification aligned with IEC-62304 and ISO-9000 processes to successfully transfer the system into manufacturing.
Sunrise Support
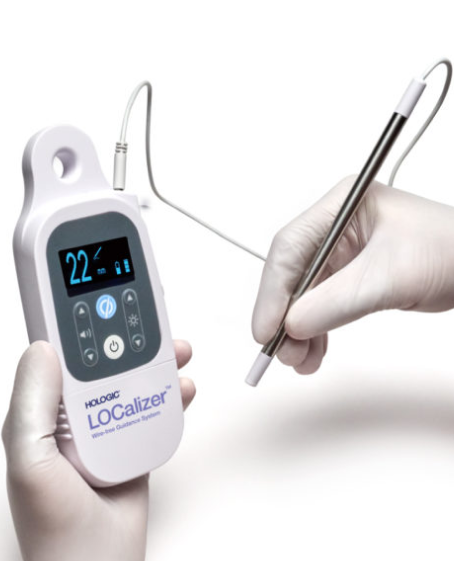
Sunrise provided support and overall management through the entire product development journey to validate and verify the system. Obsolete components were removed and new processors, sensing coiling, and display with customized iconography were added. Testing and verification aligned with IEC-62304 and ISO-9000 processes to successfully transfer the system into manufacturing.

Let’s Work Together
Let us help you solve critical challenges to change people’s lives. Partner with our team of experts passionate about applying technology to improve lives.




